Search
Name
Structure
CAS No.
Quality Standard
Application
status
Apalutamide
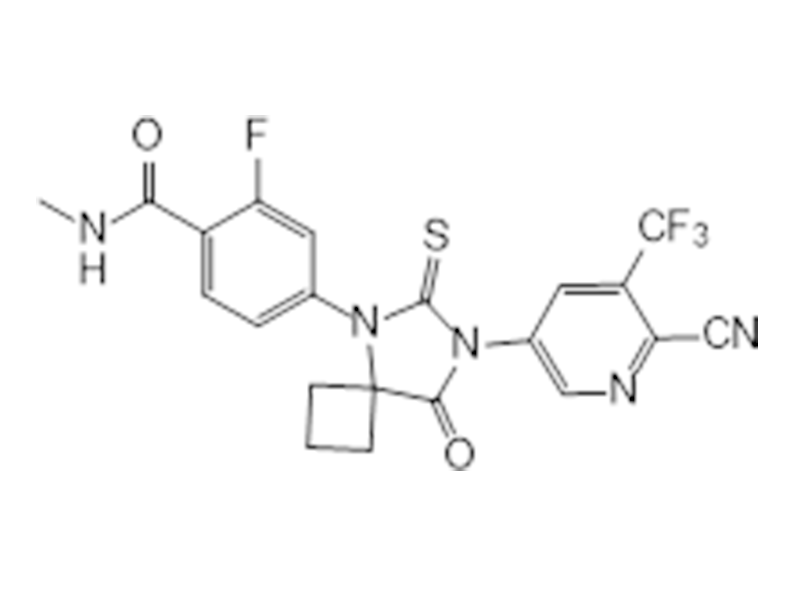

956104-40-8
In house
Prostate cancer
Commercialized//Validated
Apixaban
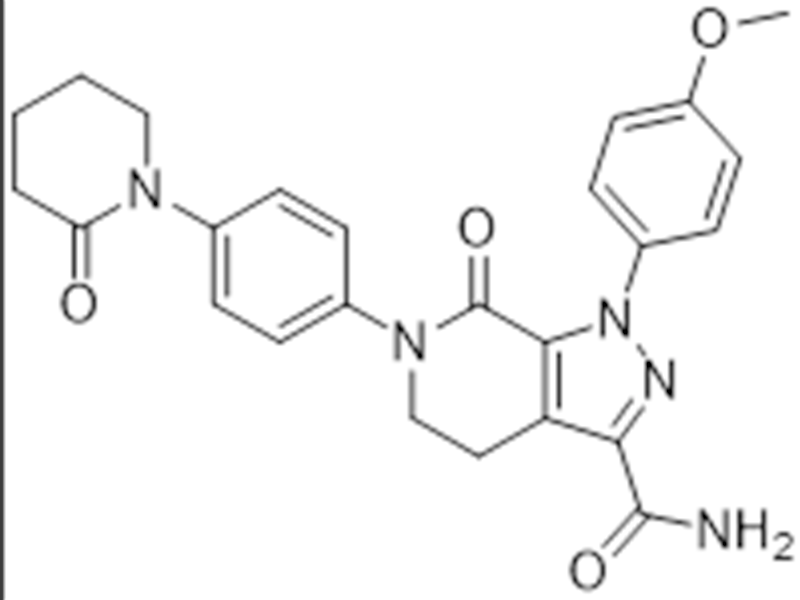

503612-47-3
In house
Anticoagulant
Commercialized//Validated
Avatrombopag maleate
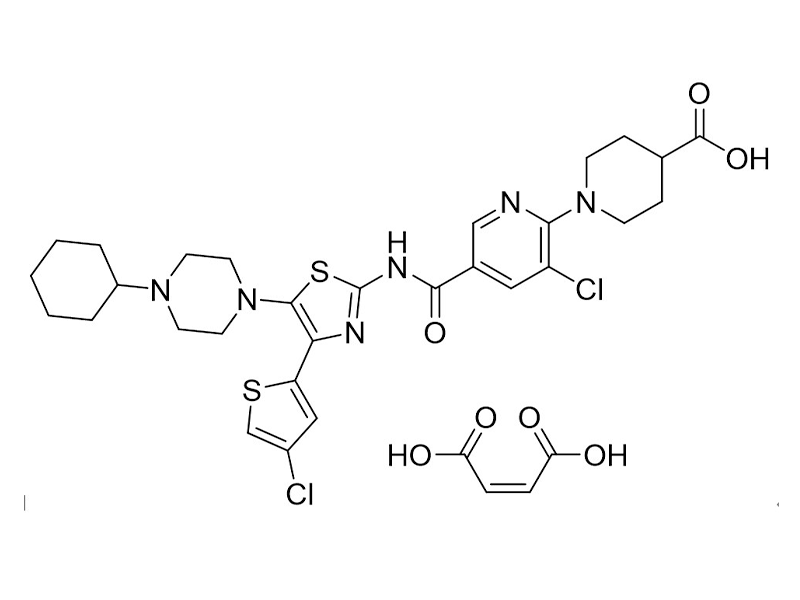

570406-98-3
In house
Antithrombocytopenics
Commercialized//Validated
Azilsartan
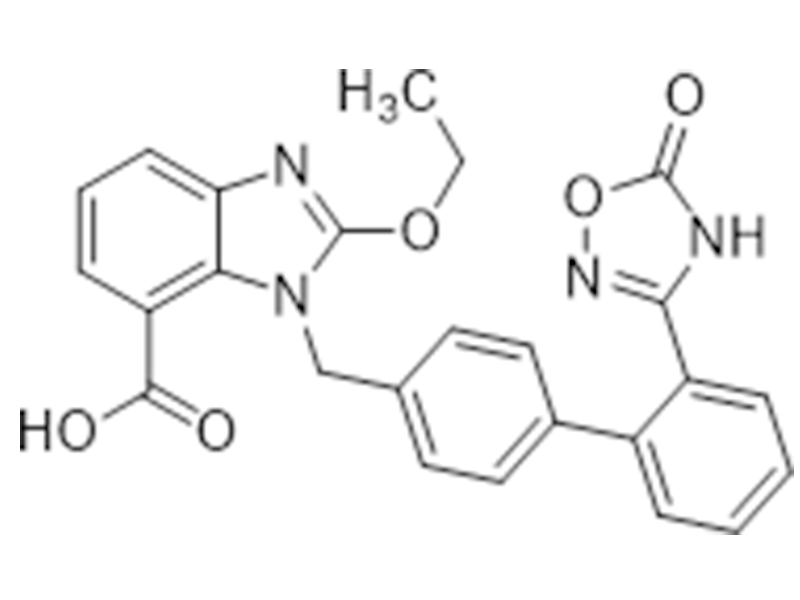

147403-03-0
In house
Antihypertensive
Commercialized//Validated
Azilsartan medoxomil potassium
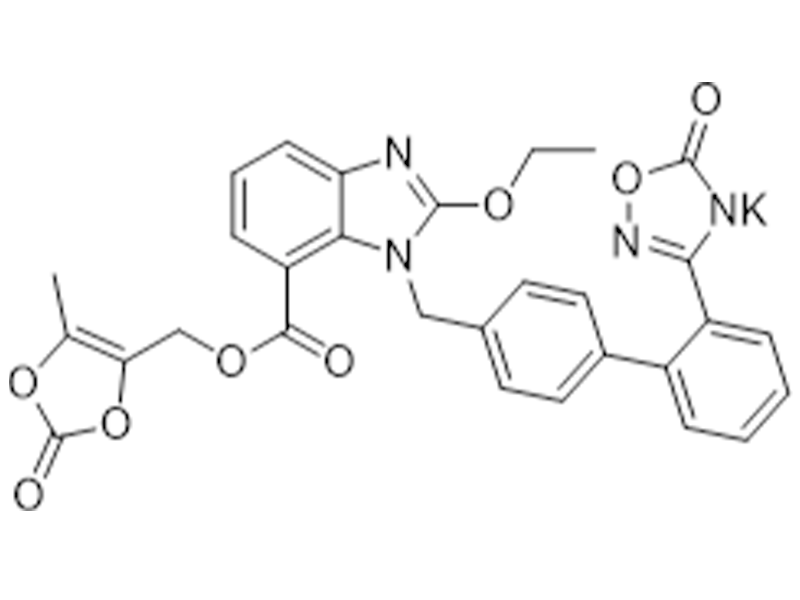

863031-24-7
In house
Antihypertensive
Commercialized//Validated
Brexpiprazole
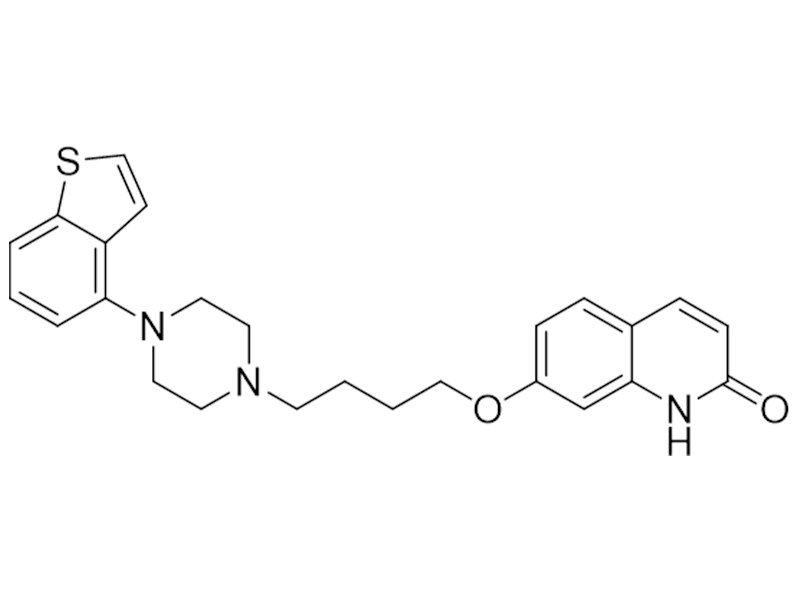

913611-97-9
In house
Antidepressants
Commercialized//Validated
Brivaracetam
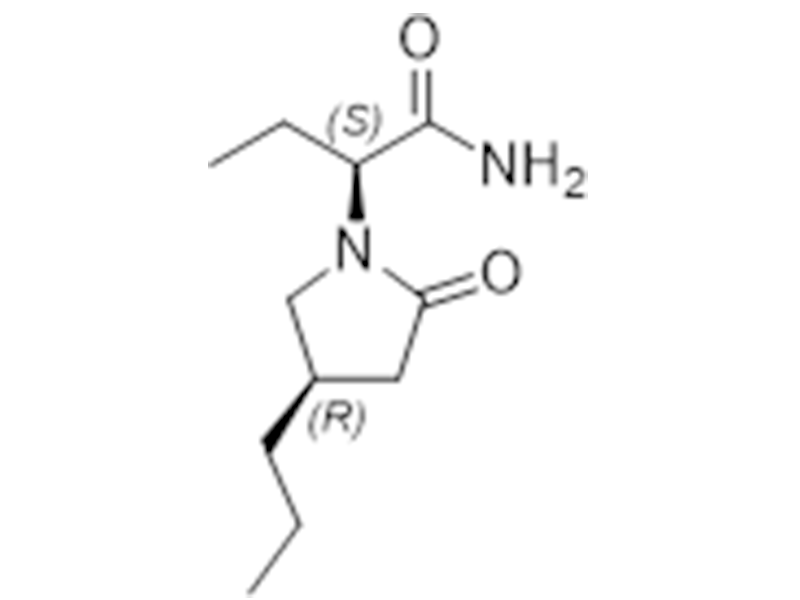

357336-20-0
In house
Antiepileptic
Commercialized//Validated
Cabozantinib malate
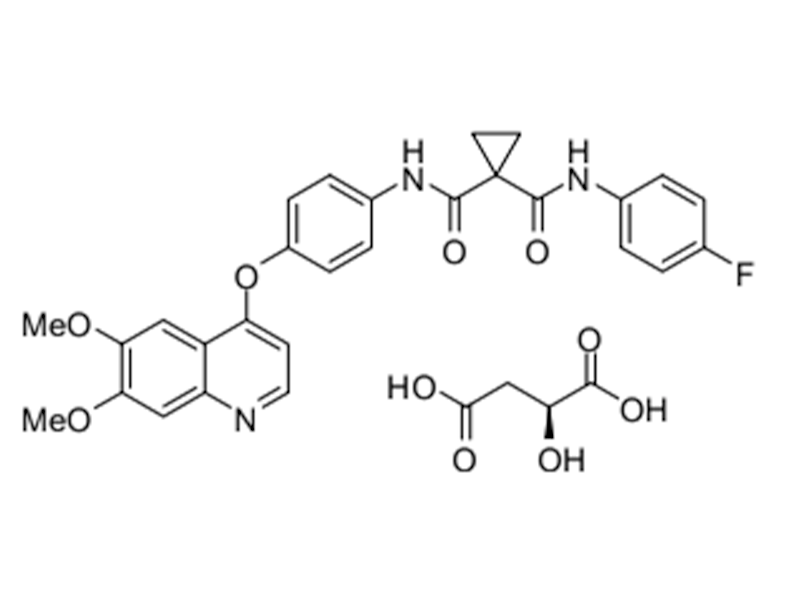

1140909-48-3
In house
Anticancer
Commercialized//Validated
Canagliflozin hemihydrate
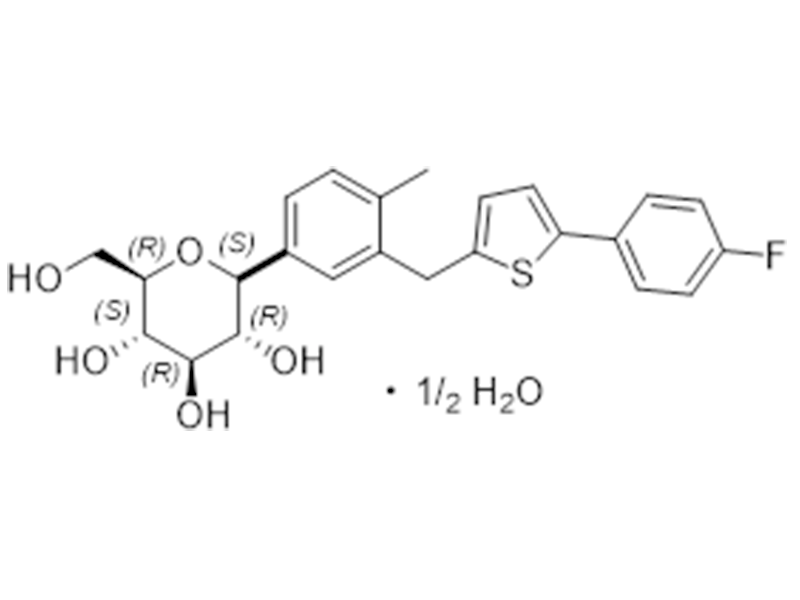

928672-86-0
In house
SGL2 Inhibitor/Antidiabetic
Commercialized//Validated
Candesartan cilexetil
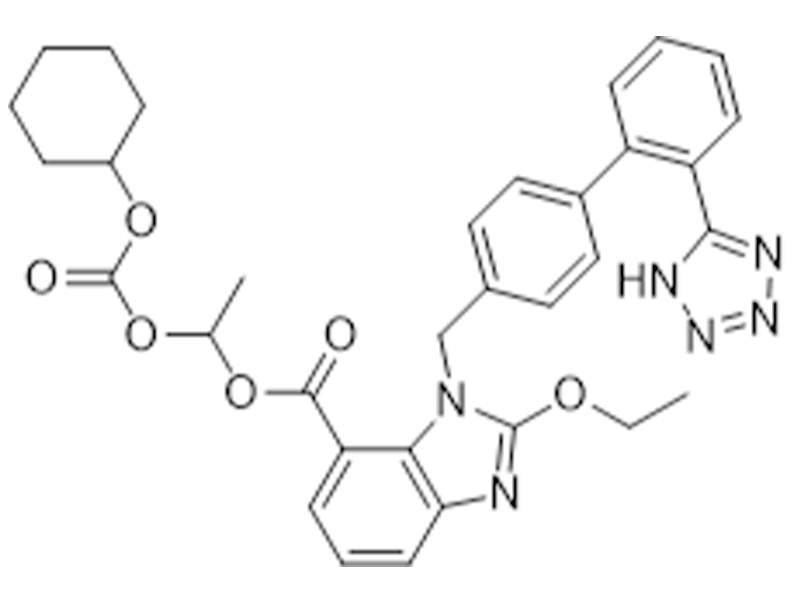

145040-37-5
USP/EP
Antihypertensive
Commercialized//Validated
Dabigatran etexilate mesylate
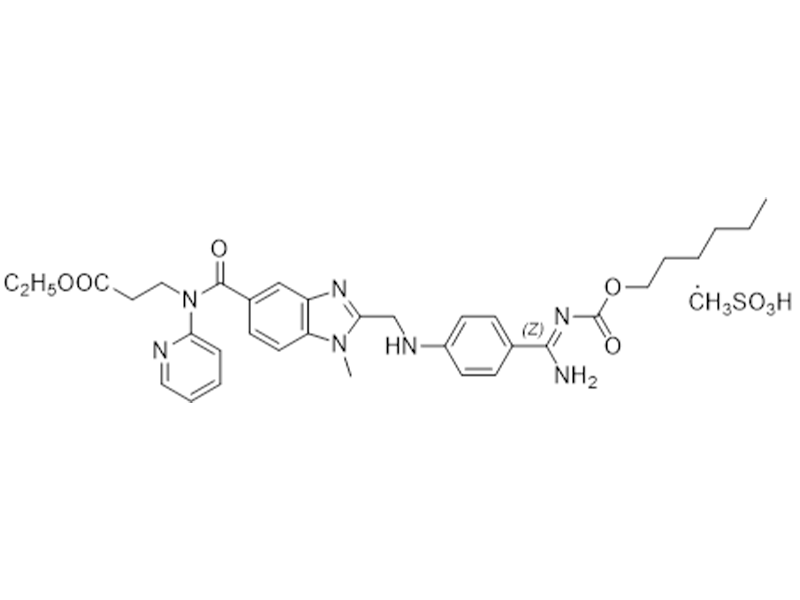

872728-81-9
In house
Anticoagulant
Commercialized//Validated
Dapagliflozin
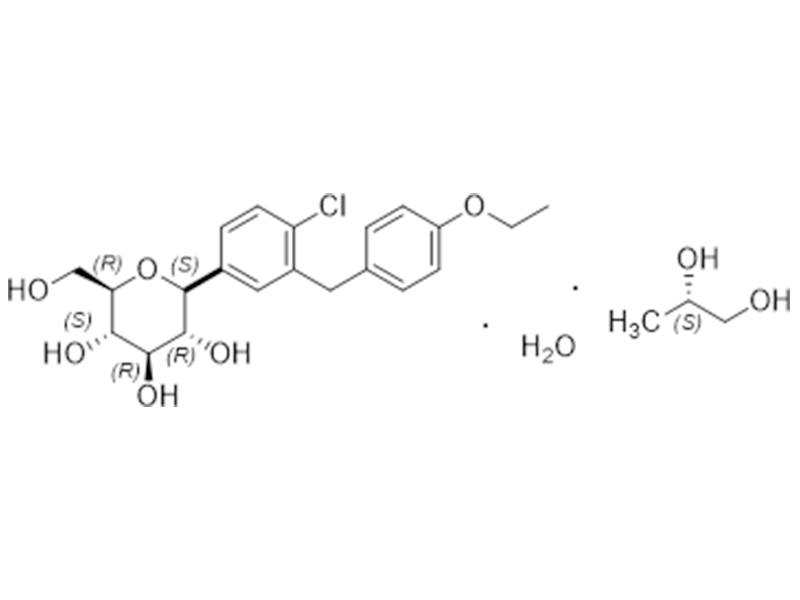

960404-48-2
In house
SGL2 Inhibitor/Antidiabetic
Commercialized//Validated
Edoxaban tosylate monohydrate
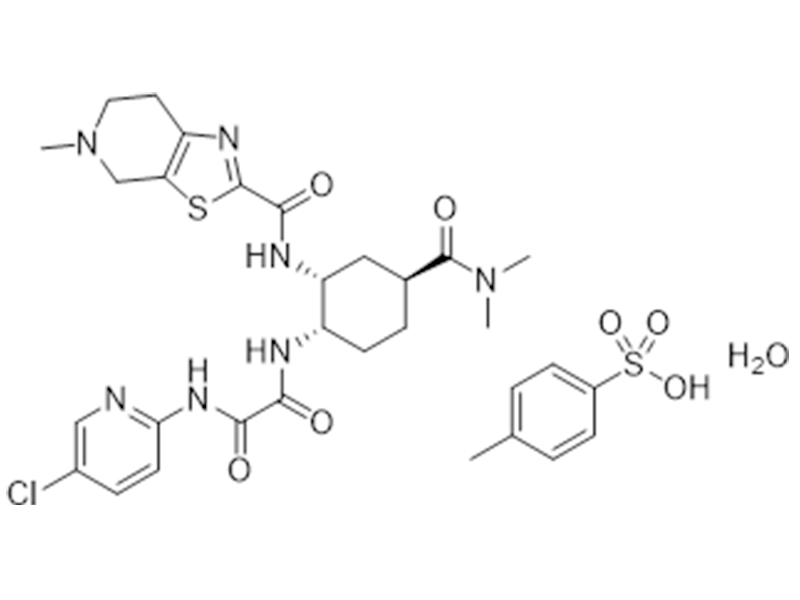

1229194-11-9
In house
Anticoagulant
Commercialized//Validated
Elagolix sodium
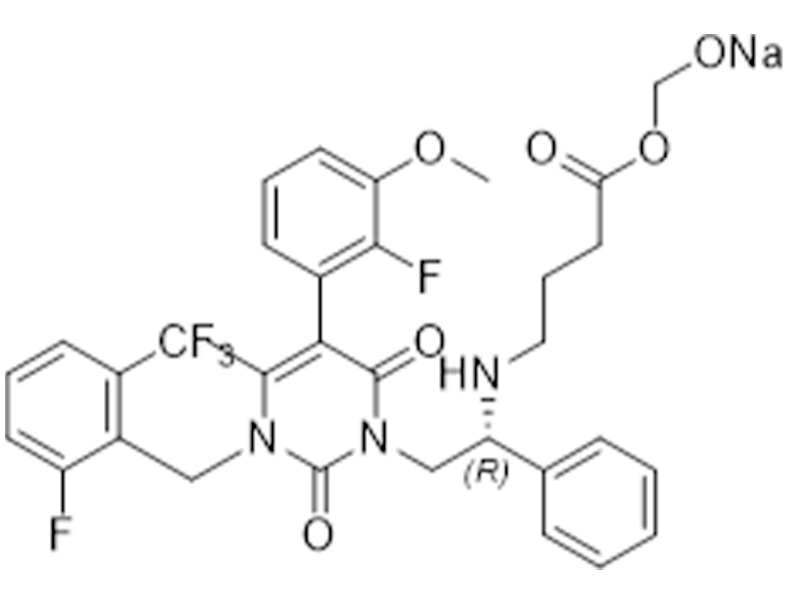

832720-36-2
In house
Pain due to endometriosis
Commercialized//Validated
Empagliflozin
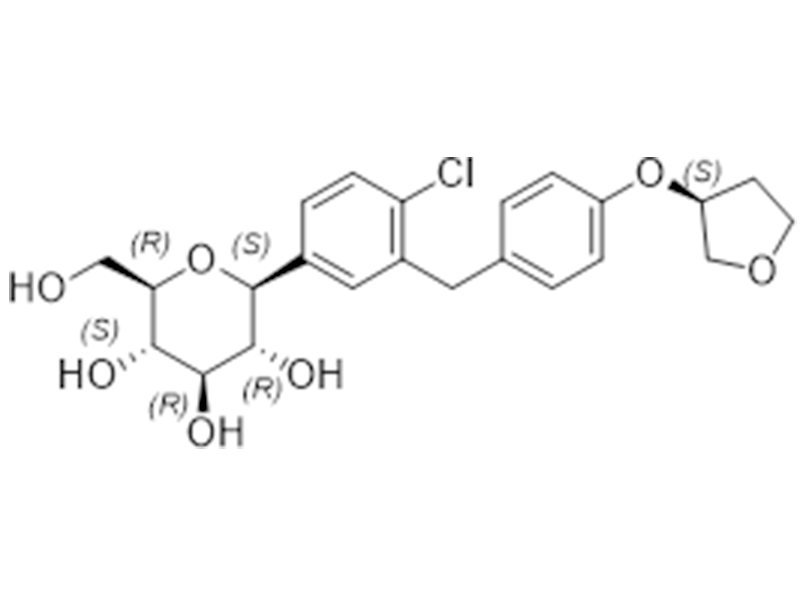

864070-44-0
In house
SGL2 Inhibitor/Antidiabetic
Commercialized//Validated
Enzalutamide
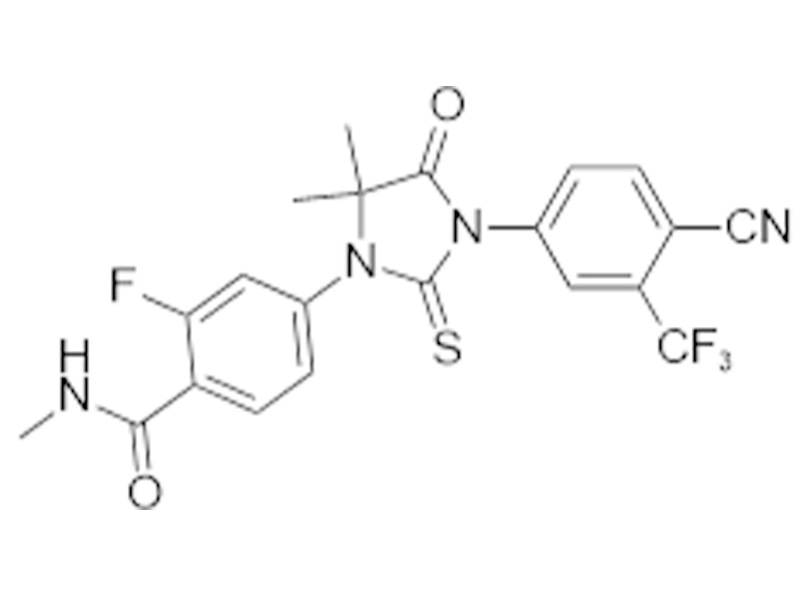

915087-33-1
In house
Anticancer
Commercialized//Validated
Ezetimibe
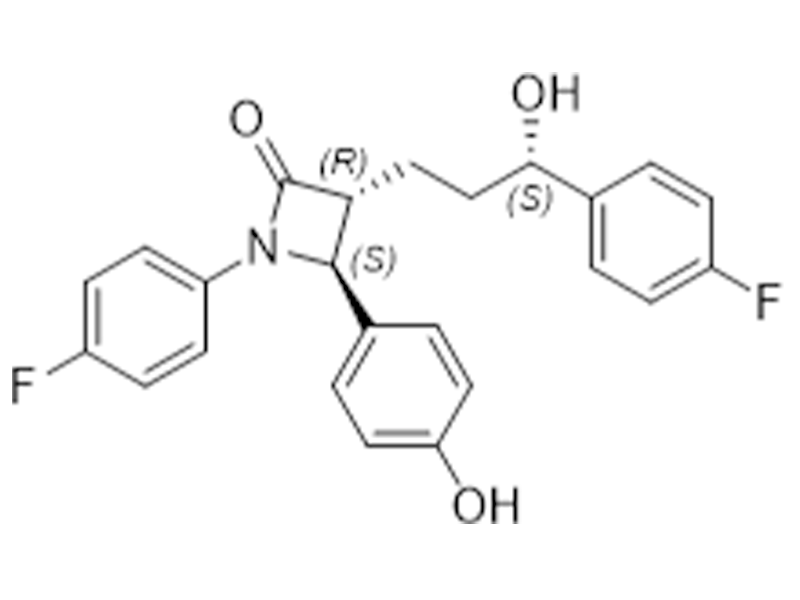

163222-33-1
USP
Cholesterol Absorption Inhibitor
Commercialized//Validated
Febuxostat
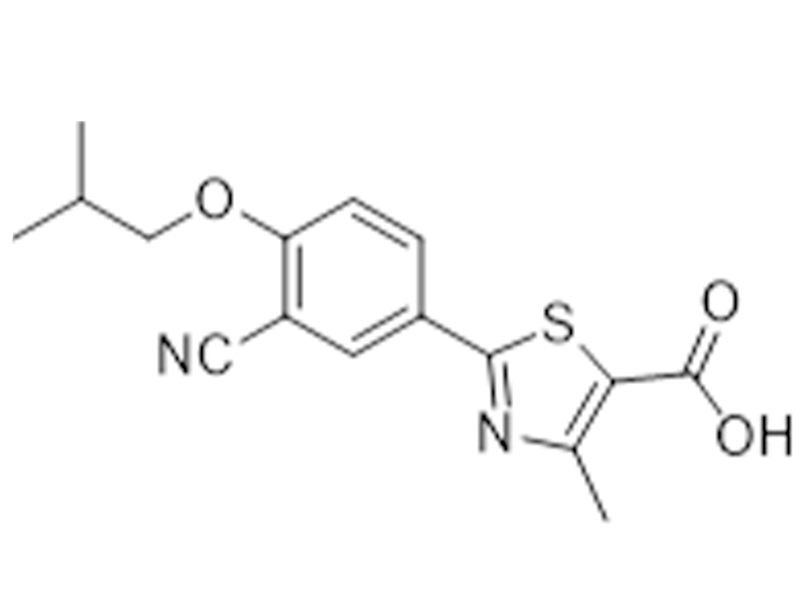

144060-53-7
In house
Antihypercholesterolemic,Antigout
Commercialized//Validated
Ibrutinib
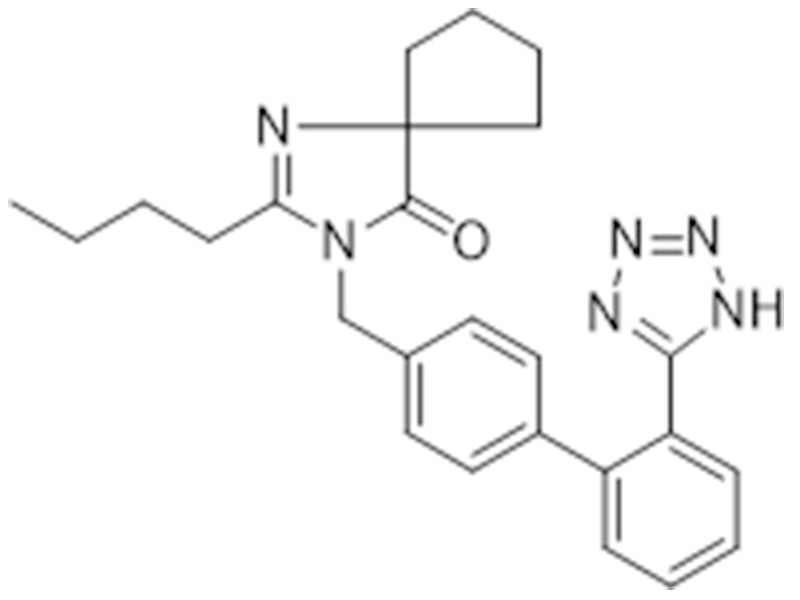

138402-11-6
In house
Antihypertensive
Commercialized//Validated
Irbesartan
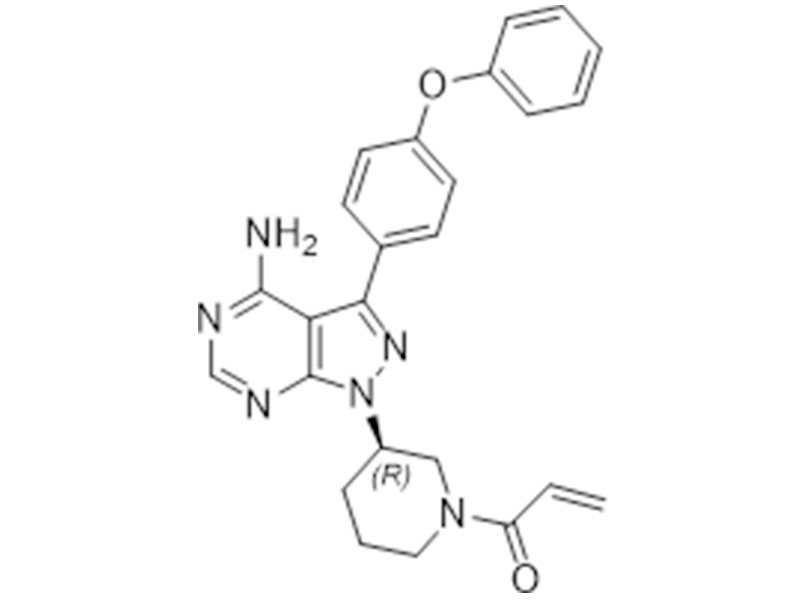

936563-96-1
USP/EP
Antiallergic, Anticancer
Commercialized//Validated
Losartan potassium
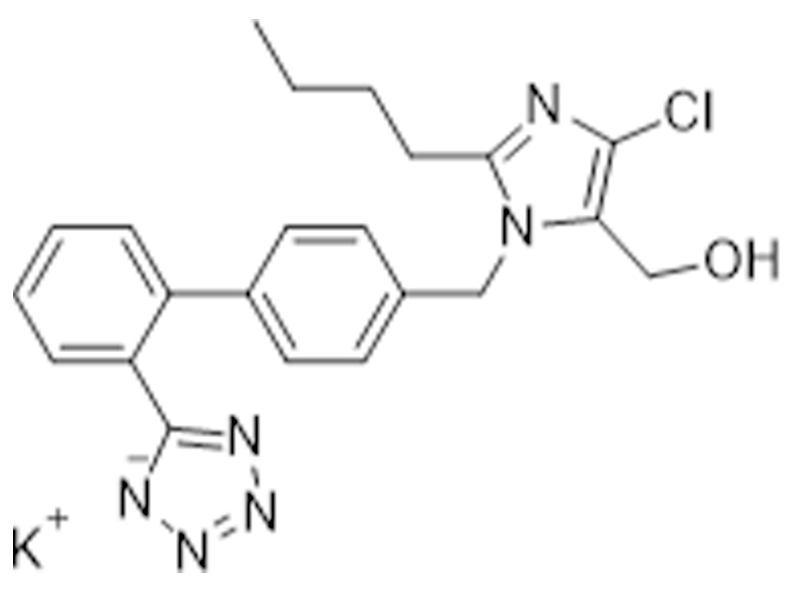

124750-99-8
USP/EP
Antihypertensive
Commercialized//Validated
Montelukast sodium
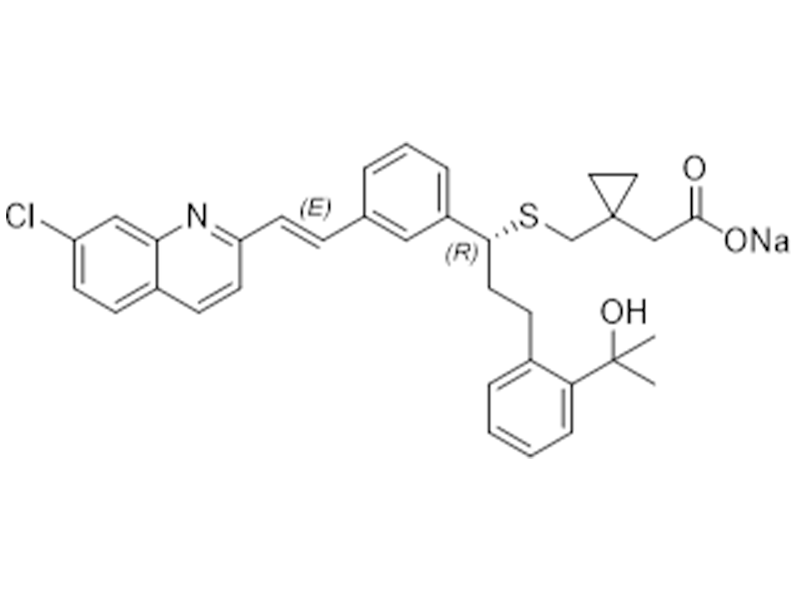

151767-02-1
USP/EP
Antiasthma
Commercialized//Validated
Nicorandil
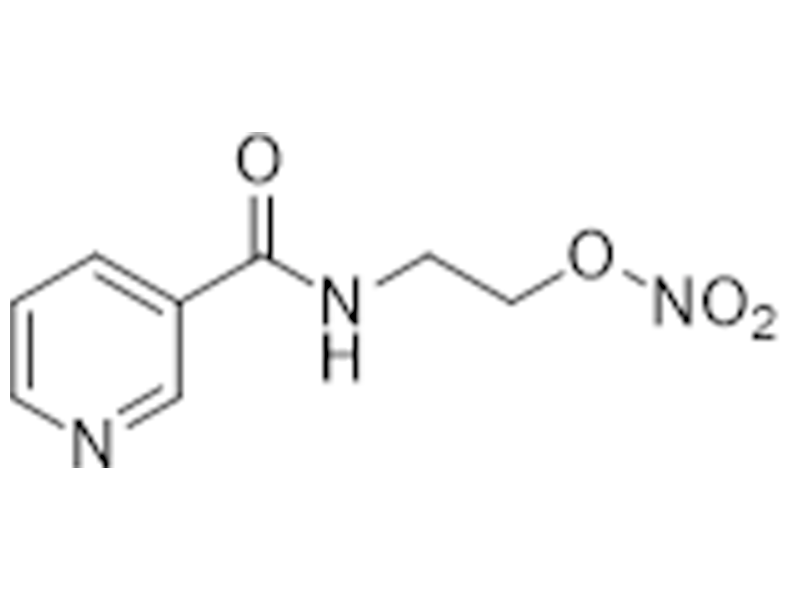

65141-46-0
JP/EP
Anti angina pectoris
Commercialized//Validated
Olmesartan medoxomil
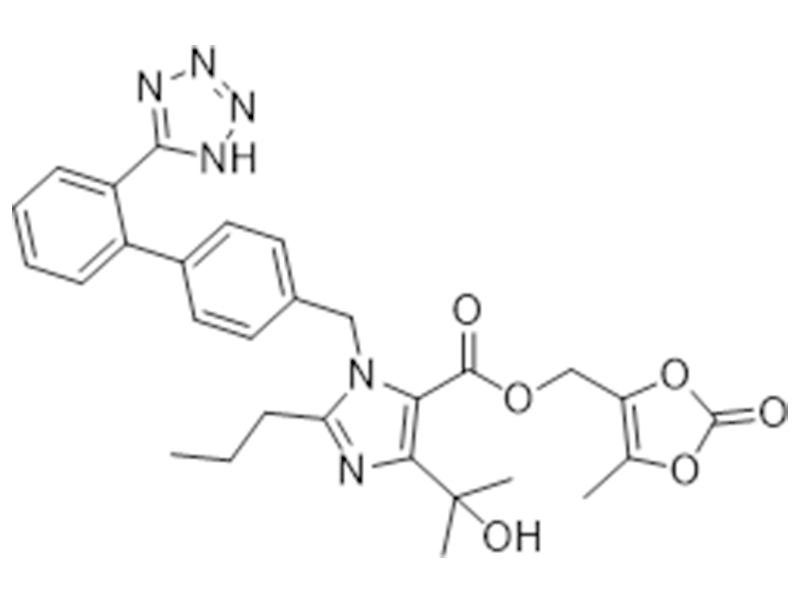

144689-63-4
USP/EP
Antihypertensive
Commercialized//Validated
Oteseconazole
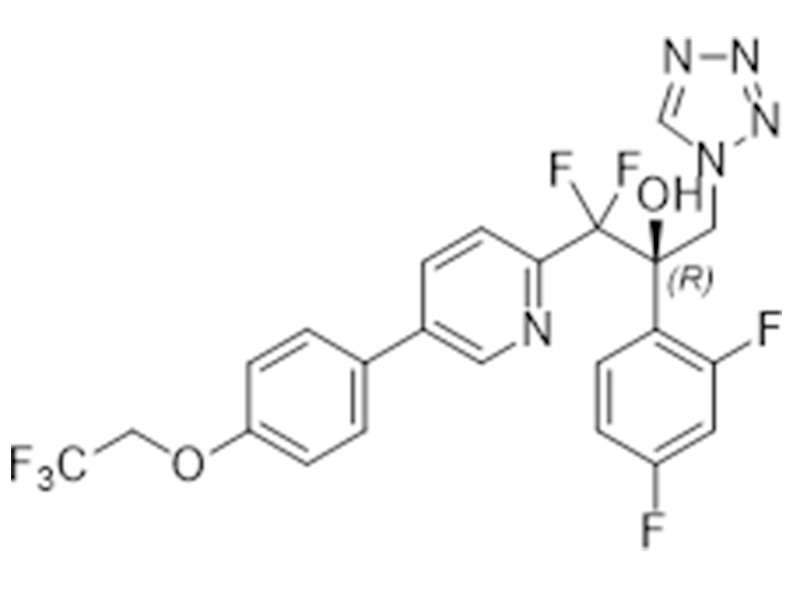

1340593-59-0
In house
Antifungal
Commercialized//Validated
Pregabalin
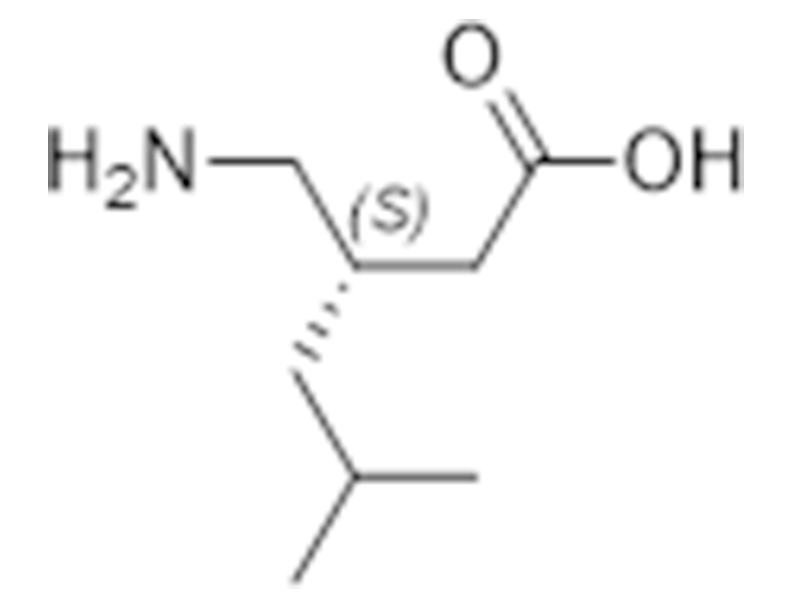

148553-50-8
In house
Antiepileptic
Commercialized//Validated
Rivaroxaban
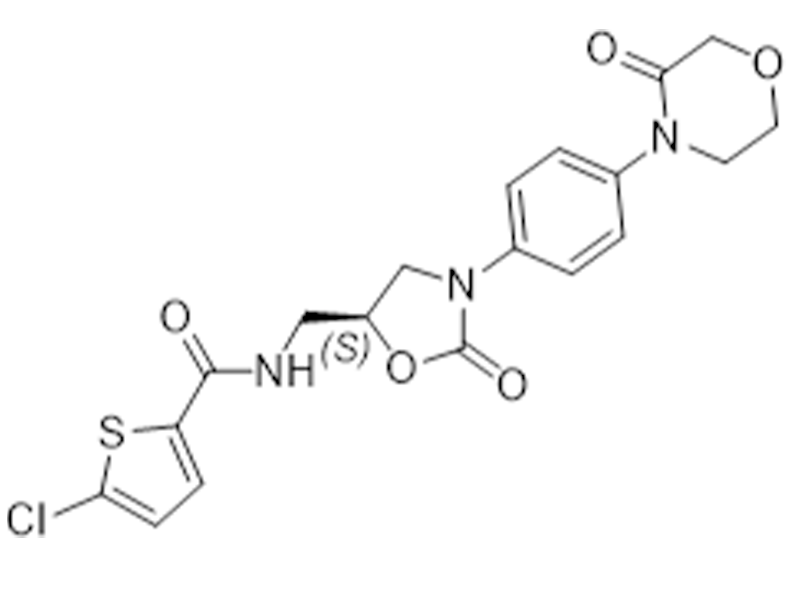

366789-02-8
EP
Anticoagulants
Commercialized//Validated
Sacubitril Valsartan Sodium
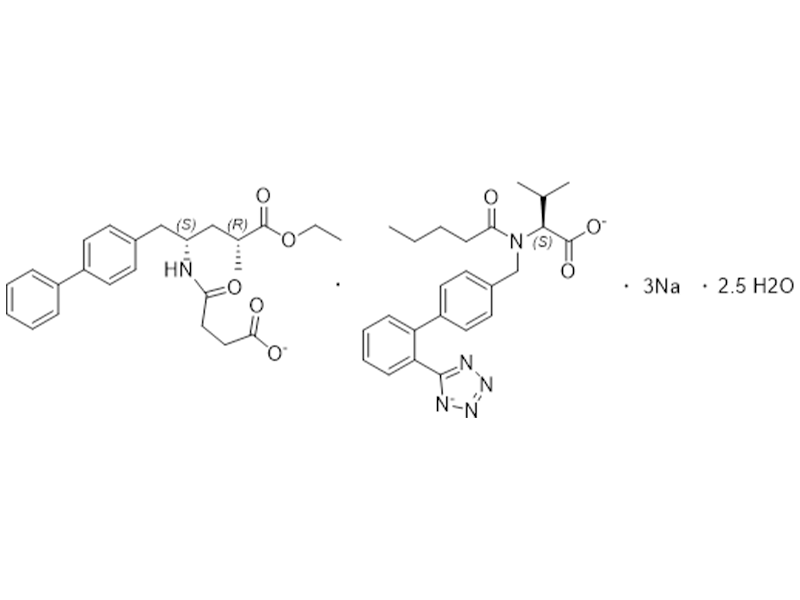

936623-90-4
In house
Antihypertensive
Commercialized//Validated
Silodosin
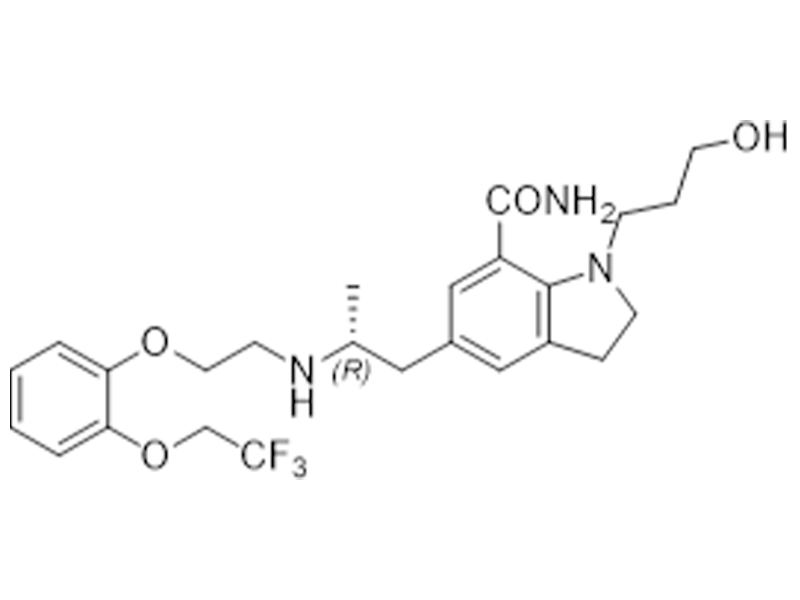

160970-54-7
JP/In house
Benign prostatic hyperplasia
Commercialized//Validated
Sitagliptin hydrochloride monohydrate
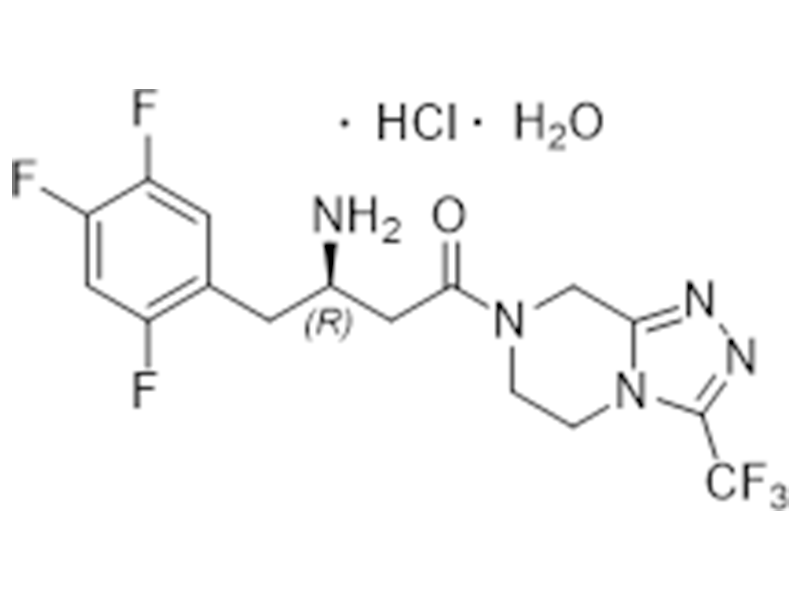

862156-92-1
In house
Antidiabetic
Commercialized//Validated
Sitagliptin phosphate monohydrate
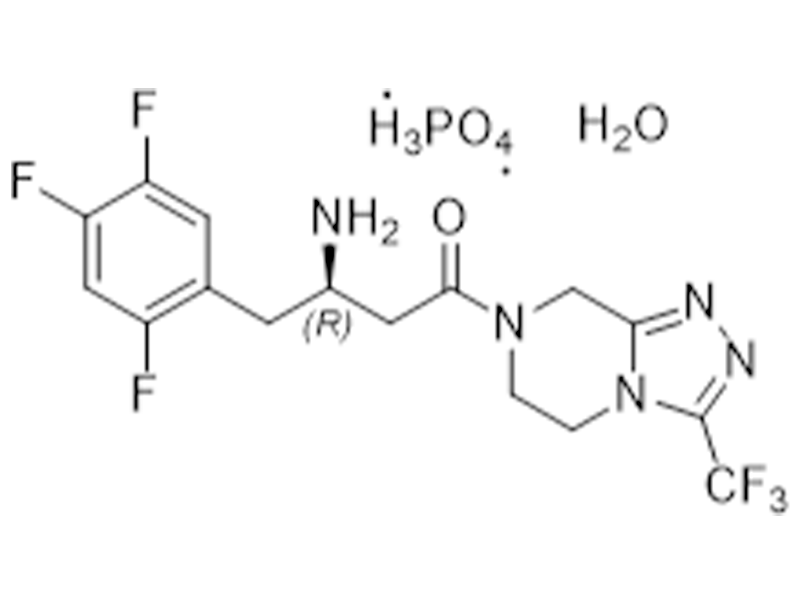

654671-77-9
USP/EP
Antidiabetic
Commercialized//Validated
Tafluprost
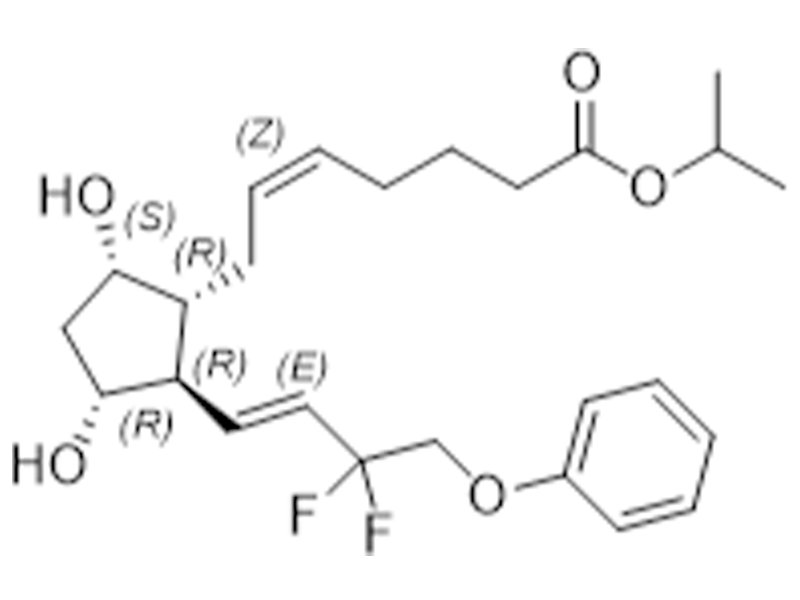

209860-87-7
In house
Antiglaucoma
Commercialized//Validated
Telmisartan
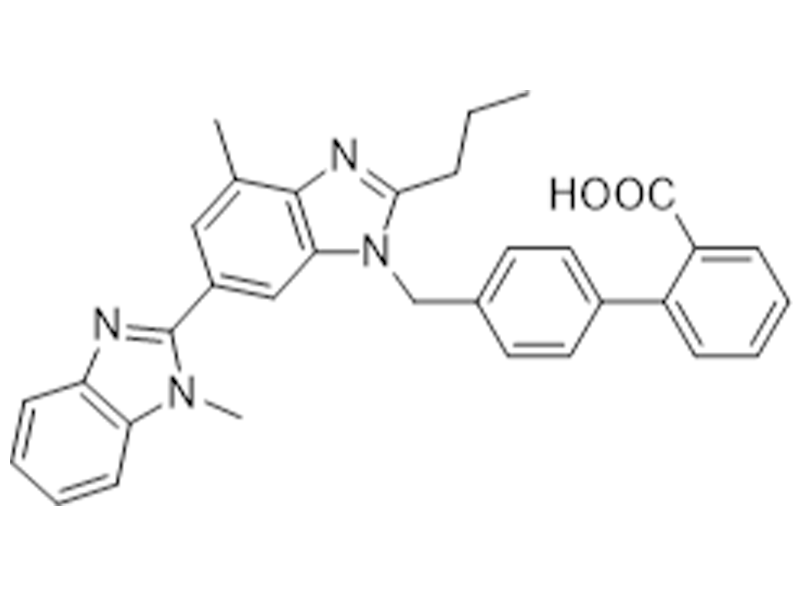

144701-48-4
USP/EP
Antihypertensive
Commercialized//Validated
Ticagrelor
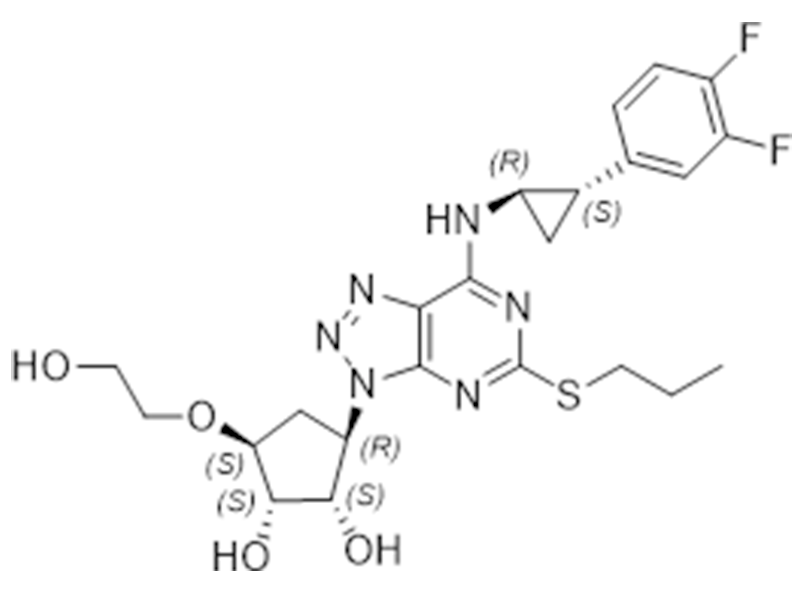

274693-27-5
EP
Antiplatelet
Commercialized//Validated
Valsartan
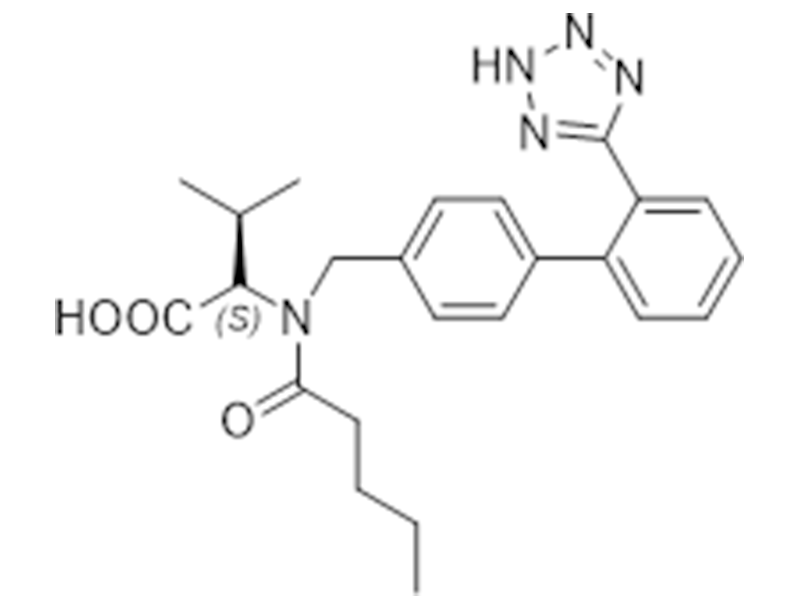

137862-53-4
USP/EP
Antihypertensive
Commercialized//Validated
Vildagliptin
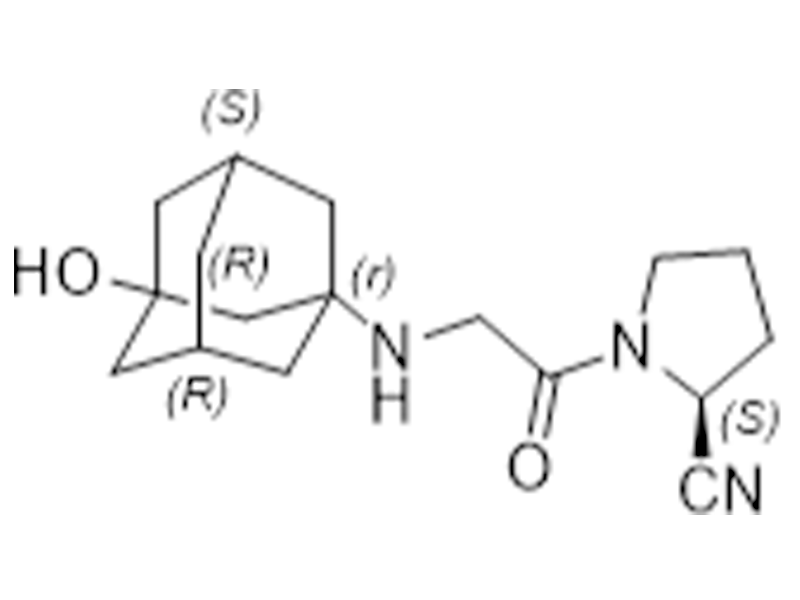

274901-16-5
In house
Antidiabetic
Commercialized//Validated
Vonoprazan fumarate
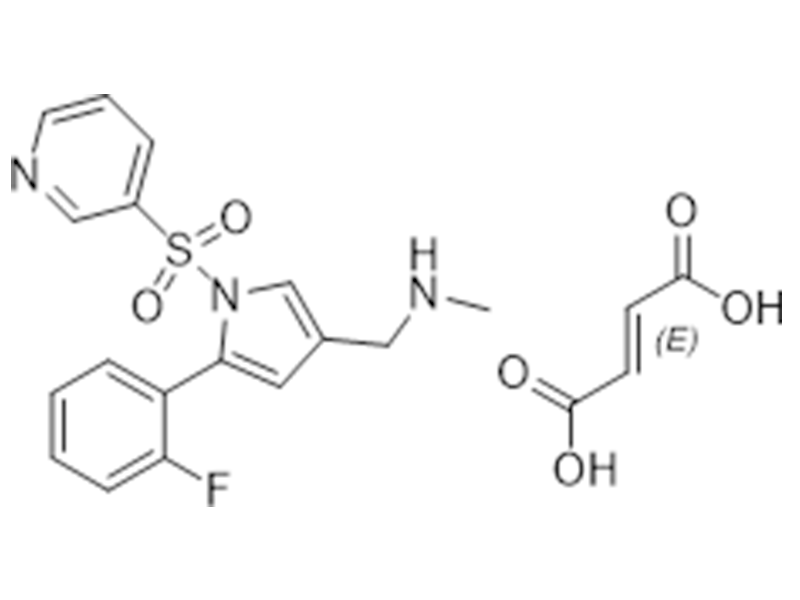

881681-01-2
In house
Antiulcer
Commercialized//Validated
Dapagliflozin Free Base
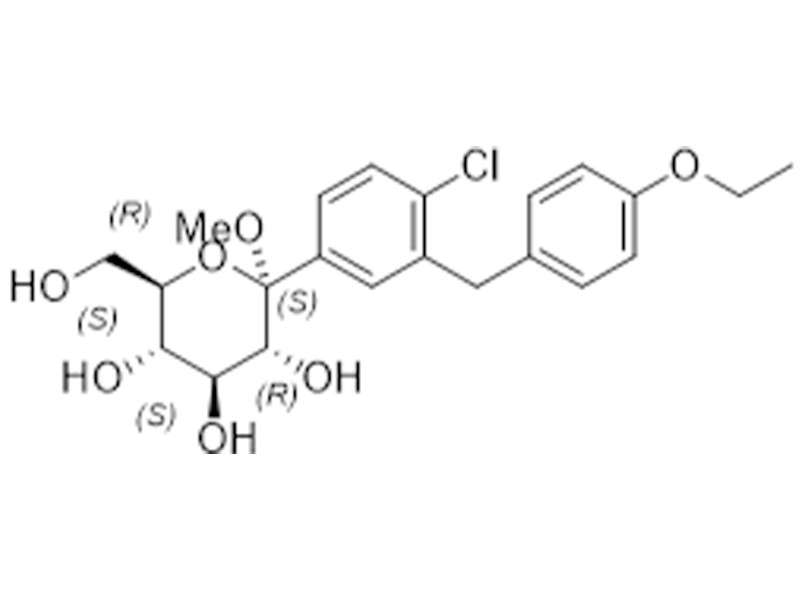

461432-26-8
In house
Antidiabetic
Commercialized//Validated
Relugolix
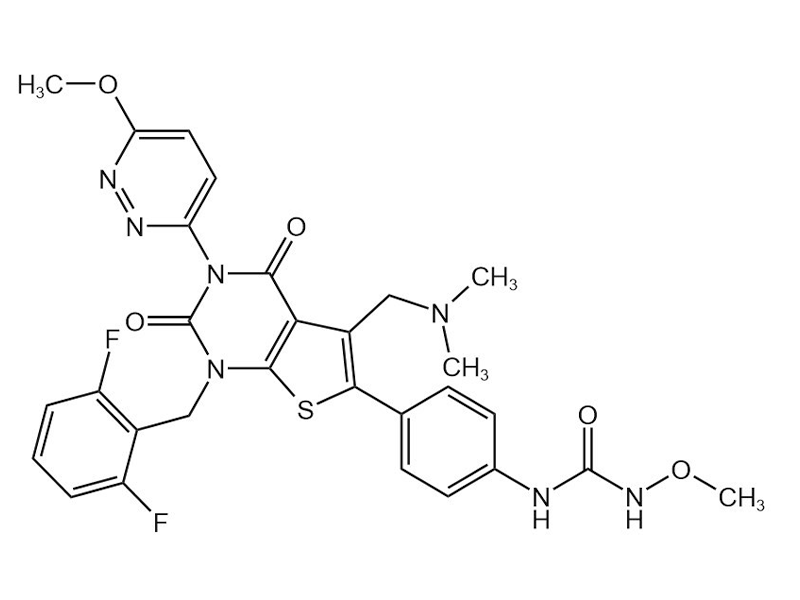

737789-87-6
In house
Endometriosis
Commercialized//Validated
Finerenone
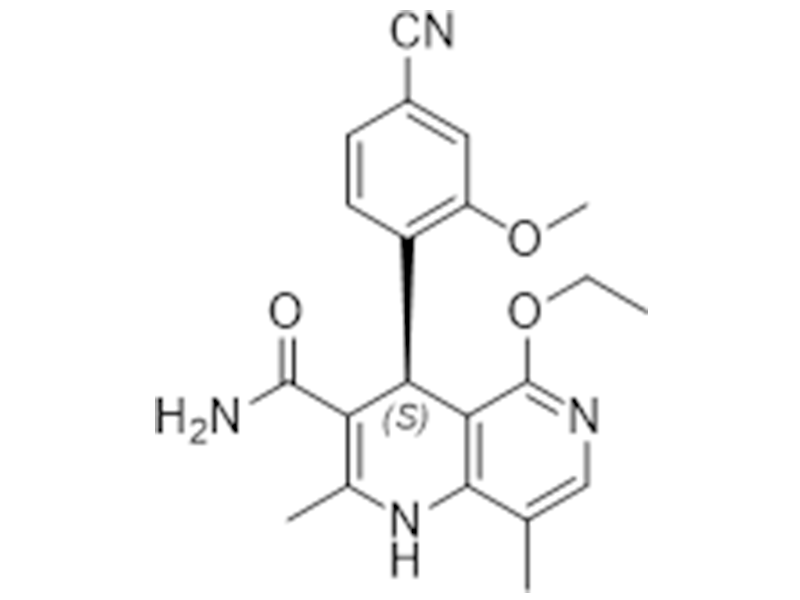

1050477-31-0
In House
Diabetic nephropathy
Commercialized//Validated
Bilastine
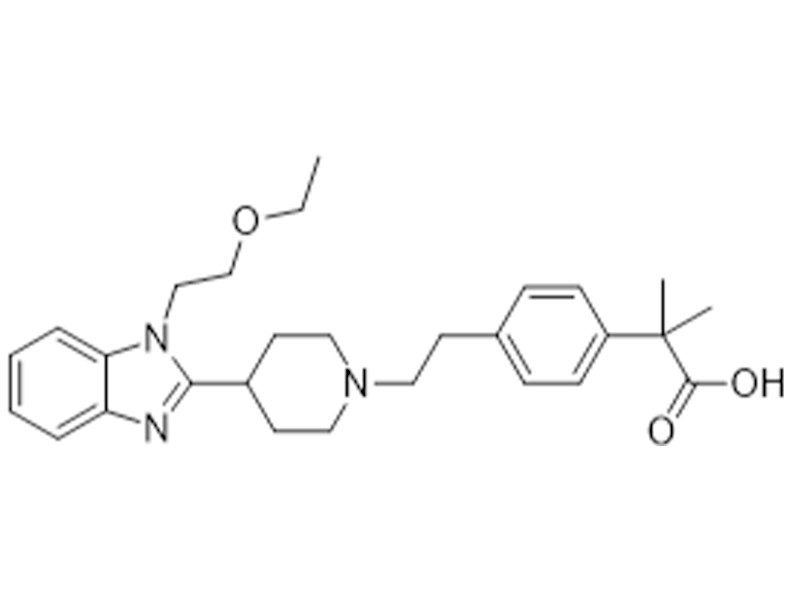

202189-78-4
In house
Antihistamine
Commercialized//Validated
Nebivolol Hydrochloride
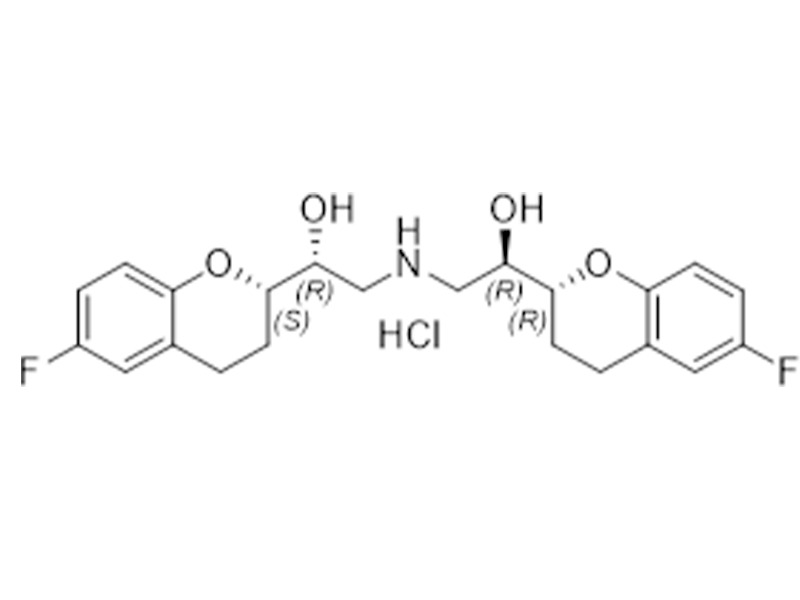

152520-56-4
CEP
Antihypertensive
Commercialized//Validated
Vibegron
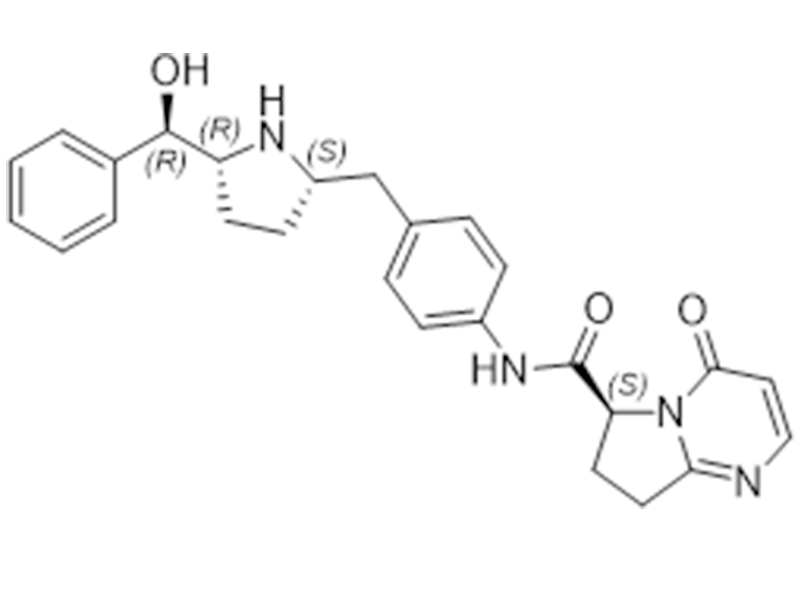

1190389-15-1
In house
Overactive bladder
Commercialized//Validated
Bempedoic Acid
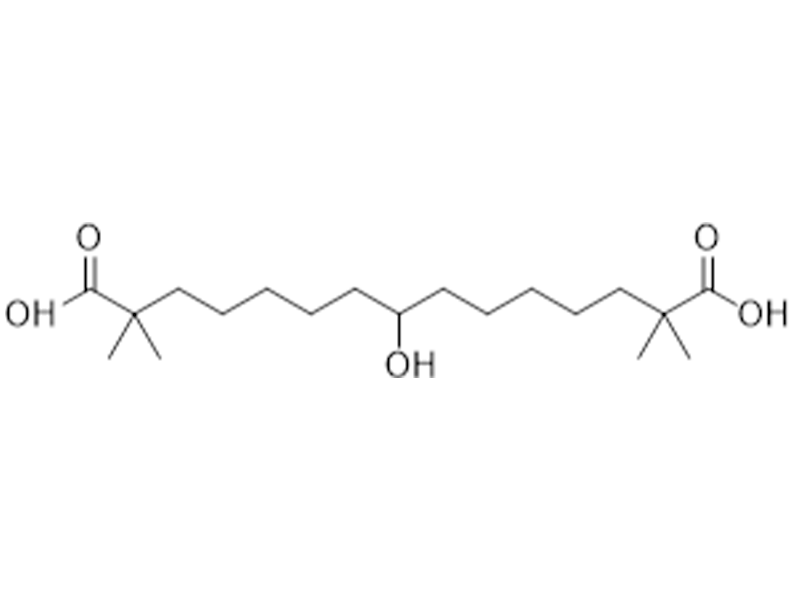

738606-46-7
In house
Antilipemic
Commercialized//Validated
Contact Information
Who We Are
Products & Services
Business Operations
CSR & Sustainability





 浙公网安备 33100302000925号
浙公网安备 33100302000925号